Register now or log in to join your professional community.

by definition
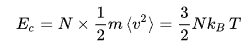
at zero temperature , particule has no kenitic energy

To say they lose their energy and stop moving at zero Kelvin, that's not right. The reason is that the atoms and molecules still have some energy (momentum) even after reaching the absolute zero temperature. That's proved experimentally.

Depending on the studies available till now, the absolute zero is the temperature at which the entropy becomes zero which means the particles won't move.

NO,BECAUSE THE THIRD LAW OF THERMODYNAMICS STATES THAT "AT ABSOLUTE ZERO ENTROPY OF A PERFECT CRYSTALLINE SUBSTANCE IS ZERO"

Although some scientist, now, think they might reach colder then the absolute zero temperature, I believe the absolute zero temperature is the coldest that can be reached and since particles will need energy to move reducing the temperature, which directly affect the energy, might case the particles to stop moving.





Do you need help in adding the right keywords to your CV? Let our CV writing experts help you.